Is C60 Graphene?
2024-04-30
Fullerene C60 Supplier
1. What is C60?
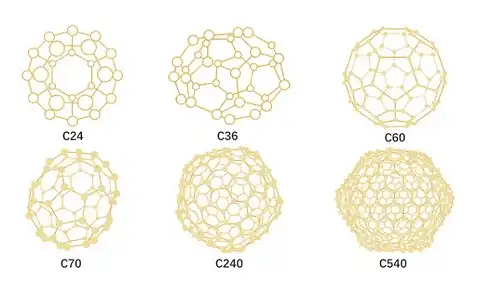
Fullerene C60 is a spherical molecule composed of carbon atoms, with a structure similar to a football. It is composed of 60 carbon atoms connected by covalent bond, so it is named C60. C60 is a new type of nanomaterial, often referred to as "carbon nanospheres" or "nanospheres". In addition to C60, there may also be C28, C32, C50, C70, C84... C240, C540, etc. with closed cage structures, collectively referred to as fullerenes. Since the discovery of fullerenes in 1985, new structures of fullerenes have been predicted or discovered, surpassing individual clusters themselves.

2. C60 Original Name
Fullerene is a new type of nanomaterial composed of carbon atoms, the most famous of which is C60 fullerene with a spherical structure. C60 fullerene is also known as carbon nanospheres or nanospheres, and its name comes from its discoverer Richard E. Sleason and Robert F. Kull's mentor Richard B. Fuller.
In the 1970s, inspired by Richard B. Fuller, Richard E. Slasen and Robert F. Kuhl began to explore the macromolecular structure constructed by pure carbon atoms. They hoped to find a new material similar to graphene. Through experiments, they successfully synthesized a new type of carbon molecule C60 in the laboratory, which is composed of 60 carbon atoms and exhibits a spherical structure, hence it is named fullerene. After synthesizing C60, scientists such as Sleason have successively synthesized other shapes of fullerenes, such as spherical molecules of different sizes such as C70, C76, and C84. The discovery of these fullerene molecules is of great significance for understanding the properties and structures of new materials.
The reason why fullerene has received widespread attention is not only due to its unique structure, but also due to its extremely high chemical and physical activity. Fullerene exhibits excellent performance in many aspects, with good photoelectric properties, mechanical strength, and biocompatibility, and is considered an important material. The discovery of fullerene has also deepened people's exploration of carbon materials, promoting the development of new material fields such as carbon nanotubes and graphene.
3. Is C60 Graphene?
No, C60 is not graphene. Both C60 (fullerene) and graphene are allotropy of carbon, but their structures are different. Graphene is composed of a planar single-layer crystal composed of carbon atoms, exhibiting a hexagonal lattice; C60 is a spherical molecule composed of 60 carbon atoms, exhibiting a football like structure. Although they are different, both have unique physical, chemical, and electrical properties and broad application prospects, which have attracted the attention of many scientific workers.
The difference between fullerene and graphene
(1). Structure
Fullerene is a spherical molecule composed of 60 carbon atoms, exhibiting a football like structure; Graphene, on the other hand, is a single-layer planar hexagonal lattice with a structure similar to a honeycomb.
(2). Physical property
Fullerenes have high chemical and physical activity, making them very suitable for use in fields such as optoelectronic devices, chemical sensors, catalysts, etc. Due to the strong van der Waals interactions between fullerene molecules, their polymers are often used in fields such as liquid crystal displays.
Graphene, on the other hand, has excellent mechanical and electrical properties and is considered a new material with high application prospects. Because graphene is a single-layer carbon atom thin sheet, it has a very high surface area, strong mechanical strength and elasticity, as well as excellent conductivity and thermal conductivity. These special physical properties make graphene widely used in fields such as electronic devices, sensors, and nanomachines.
(3). Chemical property
There are also significant differences in the chemical properties between fullerene and graphene. Fullerenes have high reactivity and can react with various compounds. Graphene, on the other hand, is highly sensitive to substances in the environment due to its single-layer carbon atom thin sheet and extremely high energy state on its surface. This sensitivity can be used to develop more efficient chemical sensors.
(4). Application direction
Due to the special properties and application prospects of fullerene and graphene, there are significant differences in their application directions. Fullerenes are mainly used in optoelectronics, chemistry, materials, biomedicine, and other fields; Graphene is mainly used in fields such as electronic devices, nanomachines, and biosensors.
Comparison between Fullerene and Other Antioxidants
|
Mainstream Antioxidant |
Advantage |
Comparison of antioxidant capacity with fullerene |
| Vitamin C |
Strong antioxidant ability |
172 times more than vitamin C |
| Coenzyme Q10 |
Good antioxidant effect |
100 times more than coenzyme Q10 |
| Plant Polyphenol |
The antioxidant effect is moderate |
More than 200 times more than plant polyphenols |
| SOD | Good antioxidant effect |
50 times more than SOD |
| Fullerene | Good antioxidant effect |
Known as the king of antioxidants |
4. C60 Application
(1). Optoelectronics: Fullerene C60 has excellent optoelectronic properties and can be used to manufacture electronic devices, solar cells, etc.
(2). Chemistry: C60 powder has a wide range of applications in the field of chemistry, such as synthesizing new polymers and adsorbing organic substances.
(3). Materials Science: Wholesale C60 powder is a new type of material that can be used to prepare high conductivity materials, reinforcement materials, etc.
(4). Life sciences: C60 Fullerene powder has good biocompatibility and can be used for the preparation of tumor treatment drugs, antioxidants, etc.









